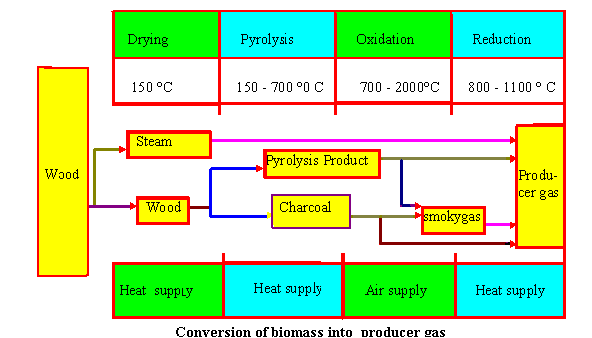
Drying
Biomass fuels consist of moisture ranging from 5 to 35%. At the tempearture above 100 o C, the water is removed and converted into steam. In the drying , fuels do not experience any kind of decomposition.Pyrolysis
Pyrolysis is the themal decomposition of biomass fuels in the absence of oxygen. Pyrolysis involves release of three kinds of products : solid, liquid and gases. The ratio of products is influenced by the chemcial composition of biomass fuels and the operationg conditions. The heating value of gas produced during the pyrolysis process is low (3.5 - 8.9 MJ/m 3 ).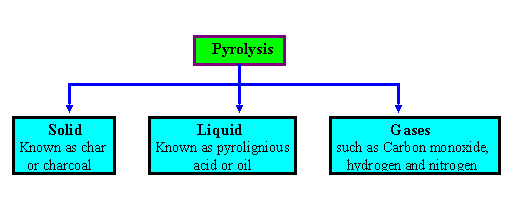
Oxidation
Introduced air in the oxidation zone contains, besides oxygen and water vapours, inert gases such as nitrogen and argon. These inert gases are considered to be non-reactive with fuel constituents.The oxidation takes place at the temperature of 700-2000o c.Heterogenous reaction takes place between oxygen in the air and solid carbonized fuel, producing carbon monoxide. Plus and minus sign indicate the release and supply of heat energy during the process respectively
C + O 2 = CO 2 + 406 [ MJ/kmol]
In reaction 12.01 kg of carbon is completely combusted with 22.39 m3 of oxygen supplied by air blast to yield 22.26 m 3 of carbon dioxide and 393.8 MJ of heat.
Hydrogen in fuel reacts with oxygen in the air blast, producing steam .
H 2 + ½ O 2 = H 2 O + 242 [ MJ/kmol]
Reduction
In reduction zone, a number of high temperature chemical reactions take place in the absence of oxygen. The principal reactions that takes place in reduction are mentioned below.
Boudouard reaction
CO 2 + C = 2CO - 172.6 [MJ/kmol]
Water-gas reaction
C + H2 O = CO + H 2 - 131.4 [MJ/kmol]
Water shift reaction
CO 2 + H 2 = CO + H 2 O + 41.2 [MJ/kmol]
Methane production reaction
C + 2H 2 = CH 4 + 75 [MJ/kmol]
Main reactions show that heat is required during the reduction process. Hence, the temperature of gas goes down during this stage. If complete gasification takes place, all the carbon is burned or reduced to carbon monoxide, a combustible gas and some other mineral matter is vaporized. The remains are ash and some char (unburned cabon)
To retun to main page click here